

In the above examples, we assumed density to be constant and the average density of the fluid to be a good representation of the density. As discussed, pressure in a fluid near Earth varies with depth due to the weight of fluid above a particular level. The pressure at any point in a static fluid depends only on the depth at that point. At any point within a static fluid, the pressure on all sides must be equal-otherwise, the fluid at that point would react to a net force and accelerate. Pressure in a static fluid in a uniform gravitational fieldĪ static fluid is a fluid that is not in motion. The weight of the fluid is equal to its mass times the acceleration due to gravity. The pressure due to the fluid is equal to the weight of the fluid divided by the area.
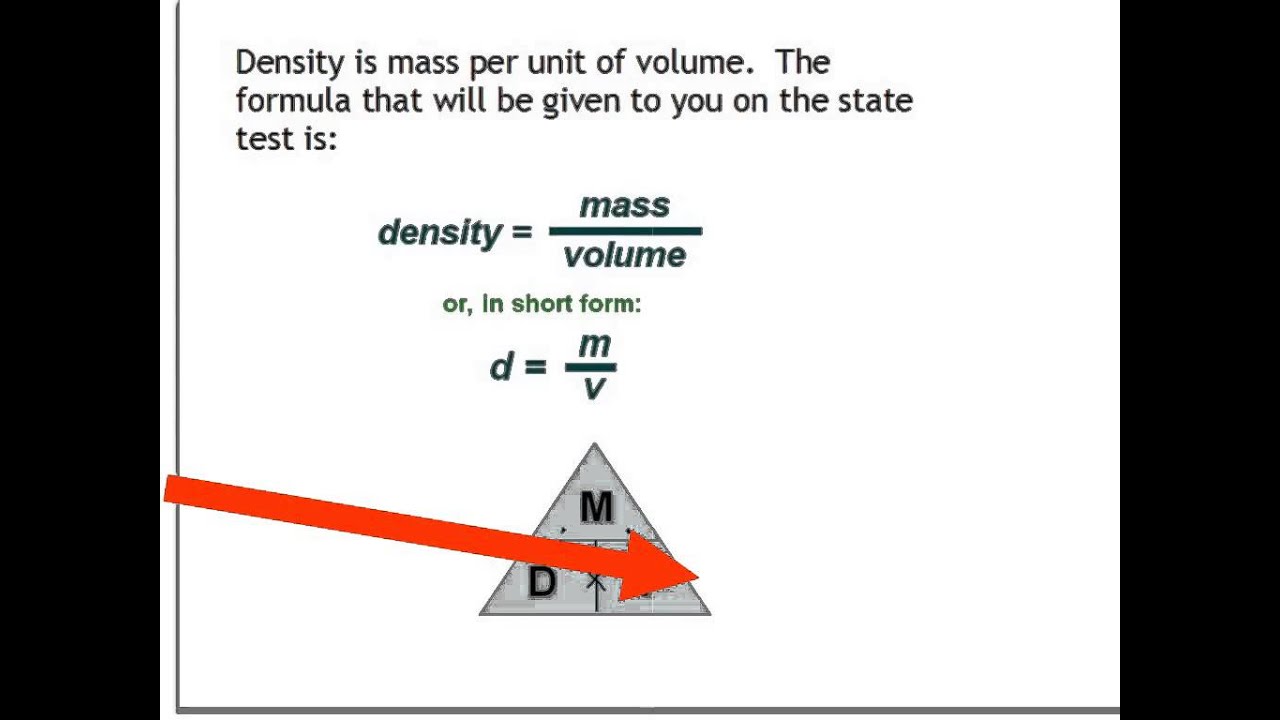
Density formula plus#
Plasma will not be discussed in depth in this chapter because plasma has very different properties from the three other common phases of matter, discussed in this chapter, due to the strong electrical forces between the charges.ġ\,\text) plus the pressure due to the weight of the fluid. At high temperatures, molecules may disassociate into atoms, and atoms disassociate into electrons (with negative charges) and protons (with positive charges), forming a plasma.
There exists one other phase of matter, plasma, which exists at very high temperatures. In this chapter, we generally refer to both gases and liquids simply as fluids, making a distinction between them only when they behave differently. When placed in an open container, gases, unlike liquids, will escape. This makes gases relatively easy to compress and allows them to flow (which makes them fluids). In contrast, atoms in gases are separated by large distances, and the forces between atoms in a gas are therefore very weak, except when the atoms collide with one another. Because the atoms are closely packed, liquids, like solids, resist compression an extremely large force is necessary to change the volume of a liquid. When a liquid is placed in a container with no lid, it remains in the container. That is, liquids flow (so they are a type of fluid), with the molecules held together by mutual attraction.
Density formula free#
This occurs because the atoms or molecules in a liquid are free to slide about and change neighbors. Liquids deform easily when stressed and do not spring back to their original shape once a force is removed. A gas must be held in a closed container to prevent it from expanding freely and escaping. (c) Atoms in a gas move about freely and are separated by large distances. Forces between the atoms strongly resist attempts to compress the atoms. (b) Atoms in a liquid are also in close contact but can slide over one another. Figure 14.2 (a) Atoms in a solid are always in close contact with neighboring atoms, held in place by forces represented here by springs.


 0 kommentar(er)
0 kommentar(er)
